Testing for predisposition to hereditary diseases
With the aim of prevention, the diseases that your genetic material predisposes you to can be identified using genetic methods. Knowledge of the elevated risk can direct your lifestyle or therapy towards prevention.
Our diagnostic laboratory provides testing for the following hereditary diseases:
- Cystic fibrosis
- Hereditary breast cancer
- Hereditary ovarian cancer
- Hereditary predisposition to thrombosis
- Lactose intolerance
The tests are performed employing state-of-the-art technology the sensitivity of which enables us to provide high-precision, reliable diagnostic results. A simple blood draw is enough for the assay.
FREQUENTLY ASKED QUESTIONS
Hereditary breast and ovarian cancer
Mutations of the BRCA1 and BRCA2 genesBreast cancer is the most common malignant tumour in women in Hungary. In the general population, women have a 10% risk of developing breast or ovarian cancer during their life.
In about 5-10% of breast cancers, an inherited gene alteration (mutation) is responsible for the disease, while this number is about 12% for ovarian cancer. In many cases, this predisposition does not only account for breast or ovarian cancers but, to a lesser extent, for tumorous degenerations of other organs (pancreas, uterus, cervix, prostate) as well.
Approximately half of the hereditary mutations responsible for predisposition to breast cancer are located in the BRCA1 gene and one-third in the BRCA2 gene. If an individual carries a mutation in either of these two genes, she has a 55-88% probability of developing breast cancer until the age of 75 and a 26-48% probability of developing ovarian cancer.
The high probability of the development of the diseases justifies the mutation analysis of the BRCA1 and BRCA2 genes in individuals at risk. At present, it is recommended to perform genetic screening of the family if one of the conditions below applies:
- presence of breast cancer in both breasts
- multiple primary breast cancers or presence of both breast and ovarian cancer
- male breast cancer
- presence of one of the three cases mentioned above in a first-degree relative
- the development of breast cancer before the age of 40
- one or more relatives with breast cancer and one or more relatives with ovarian cancer from the same branch of the family
- a verified BRCA1/2 mutation-carrier relative
The analysis can verify the hereditary nature of an already existing disease. At the same time, if the genetic predisposition caused by the mutation is verified with the analysis in relatives who do not present clinical symptoms yet, early screening for the tumour, necessary surgical intervention, and treatment can be performed.
Map of the spread of ovarian cancer
Age-standardized deaths due to ovarian cancer per 100 000 inhabitants in 2004.
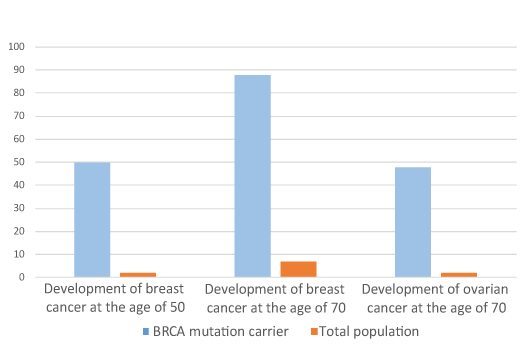
To what extent does a BRCA mutation increase the risk of breast and ovarian cancer?
The degree by which a BRCA mutation increases the risk of cancer should be interpreted for everyone separately and is influenced by many factors together. The level of risk depends on the position of the mutation within the gene, the age, the number of pregnancies, etc. The average elevation of risk for carriers of BRCA is demonstrated in the figure.
FREQUENTLY ASKED QUESTIONS
- Breast cancer at the age of 35 or earlier.
- Two or more first-degree relatives with breast cancer (average age <50) or ovarian cancer (at any age).
- Three or more close relatives with breast and/or ovarian cancer within two generations (at least one cancer under the age of 50).
- Male breast cancer (at any age).
- Detected BRCA mutation in the family
Cystic fibrosis
Mutations of the CFTR geneCystic fibrosis (CF, cystic fibrosis, mucoviscidosis) is one of the most prevalent congenital diseases, which is caused by alterations (mutations) in the CFTR (cystic fibrosis transmembrane conductance regulator) gene.
In Hungary, one in every 28 persons carries a defective copy of the CFTR gene. To date, more than 1500 alterations have been identified in the CFTR gene. Mutation ΔF508del should be highlighted since this deletion is responsible for the clinical aspects of the disease in approximately two-thirds of the patients. The remaining one-third of the mutations is dispersed in the gene.
The prevalence of CF justifies the mutational analysis of the CFTR gene. This test, as yet, is not included in routine neonatal screening in Hungary, but several developed countries have already incorporated it in their routine neonatal screening.
In CF patients the CFTR protein is missing or malfunctioning leading to the following symptoms:
- In the pancreas: digestive problems.
- In the respiratory system: chronic coughs and bouts of coughing, recurrent and chronic pneumonia. Respiratory difficulties develop; thick mucus is deposited in the airways, and the lung does not get sufficient oxygen, which leads to its gradual necrosis.
- In the epididymis: due to the obstruction of the epididymis ducts, 95% of the male patients are infertile.
- The thick mucus blocks the exit ducts of the exocrine glands as demonstrated by a change in mucus production.
- In newborns, the disease is indicated by meconium ileus, that is, bowel obstruction due to the thickening of the bowel content.
- Protein deficiency
- Symptoms of deficiency of fat-soluble vitamins (A, D, E, K)..
- Inflammatory alterations are typical in almost all of the glands.
The CFTR Diagnostic Kit developed in our lab enables the analysis of the whole gene, that is, it is capable of detecting all of the mutations.
Child suffering from cystic fibrosis
Babies suffering from mucoviscidosis are born with normal lungs, but the glands of the mucous membranes continuously secrete thick mucus, which blocks the alveoli, and the respiratory surface decreases significantly. The thick mucus cannot clear up the normal way, so the little patients try to get rid of it with long bouts of coughing. At the beginning, the cough is dry, intermittent, later it seems to clear up. The thick mucus is a hotbed for different upper respiratory tract pathogens. Staphylococcus aureus and Pseudomonas species are the most frequent causes of infection. Pseudomonas aeruginosa is the most dangerous pathogen, which can cause fatal infections.
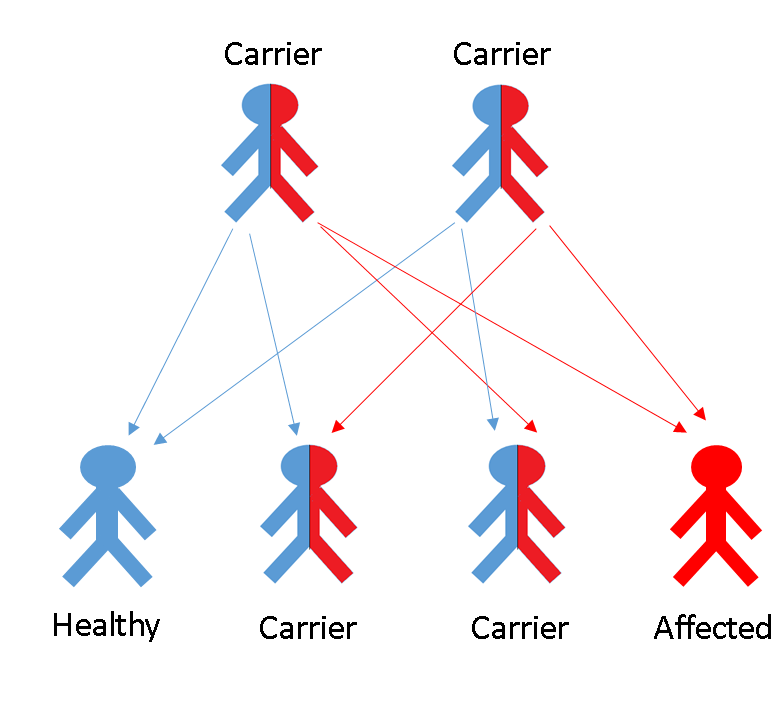
Inheritance of the disease from parents to children
If, in a given case, both parents carry a mutation in their CFTR genes they have a 25% probability that their child will be ill.
FREQUENTLY ASKED QUESTIONS
Genes predisposing to thrombosis
Venous thrombosis is a multifactorial disease with serious consequences, that is, it is caused by a combination of genetic and environmental factors. The Leiden mutation (or activated protein C resistance) is a frequent genetic alteration causing blood clotting disorder. Hereditary predisposition to thrombosis can be linked to this alteration in 80% of the cases. Due to the mutation, the patients’ blood have an increased tendency to coagulate, resulting in a predisposition to thrombosis - the blocking of a blood vessel by a clot.
This alteration is very frequent in Hungary: 10% of the population carries it. This means that only one of the two copies of the gene harbours the mutation; still, the predisposition to thrombosis increases already 8-fold. If both copies of the gene carries the mutation, the risk increases 80-fold, provided there are no further risk factors.
The mutation can be detected in 20% of the thrombosis cases of unknown cause and in 60% of the cases of thrombosis developing during pregnancy. Furthermore, this genetic alteration may be associated with recurrent miscarriages and complications of pregnancy.

The likelihood of thrombosis, besides the genetic causes, is also increased by environmental and lifestyle factors such as the use of hormonal contraceptive pills, obesity, smoking, diabetes, or sedentary life style. But pregnancy, a natural condition, may also significantly increase the risk, and thrombosis may even have consequences that endanger the foetus. In such cases, the gynaecologist should be aware of the presence of the mutation, because administration of vitamin K antagonists is strictly contraindicated during pregnancy.
The Leiden mutation (synonyms: thrombophilia, activated protein C resistance) is a blood clotting disorder of genetic origin. Due to the mutation, the degradation of factors enhancing blood clotting slows down, and thus the patients’ blood have an increased tendency to coagulate, resulting in a predisposition to thrombosis - the blocking of a blood vessel by a clot. Hereditary predisposition to thrombosis can be linked to this alteration in 80% of the cases. In the Leiden mutation, the predisposition to thrombosis is already apparent in the heterozygous form (when only one of the two copies of the gene carries a mutation) and is even more explicit in homozygous form (when the mutation is present in both copies). 10% of the healthy Hungarian population is heterozygous for the Leiden mutation, and a few per thousand are homozygous. Individuals carrying the mutation in a heterozygous form have an approximately 8-fold higher risk, while those carrying it in a homozygous form have an approximately 80-fold higher risk of deep vein thrombosis compared to the average population, provided there are no further risk factors.
It is essential that in cases of thrombosis occurring at young age with no known cause and/or in cases of recurrent thrombosis testing for the Leiden mutation should by all means be performed. If the patient has children or his/her parents are alive, they should also be tested.
Mutation of prothrombin or coagulation factor II is the second most common cause of hereditary disposition to thrombosis. Due to the alteration, the prothrombin level is elevated and, as a result, the activity of the coagulation system increases leading to a higher probability of thrombosis. This gene alteration affects 2% of the average population and increases the risk of deep vein thrombosis approximately 3-fold. The risk of developing myocardial infarction is 4-fold higher in women carrying the mutation in a heterozygous form compared to those who do not carry it, and it is further increased by other risk factors (smoking, contraceptive pills). The homozygous form of the mutation is very rare but, according to some studies, this mutation may be responsible for recurrent pregnancy losses.
Screening for the mutation is recommended in cases of brain blood vessel blockage (stroke) at a young age, in women developing myocardial infarction, after recurrent pregnancy losses, or if there is a verified prothrombin mutation-carrier relative in the family.
Mutation in the MTHFR (methylenetetrahydrofolate reductase) gene results in a hereditary elevation of the blood homocysteine level. Elevated blood homocysteine levels, which lead to an almost 3-fold increase in the risk of thrombosis compared to individuals with normal blood homocysteine level, can be found in 5-10% of the population. Elevated homocysteine level is a known risk factor of arterial and venous thrombosis, loss of pregnancy, and neural tube closure defects in the foetus. Lack of folic acid increases the probability of developing elevated homocysteine levels (hyperhomocysteinemia); thus, increased folic acid intake can reduce blood homocysteine levels. Testing the mutation is recommended in cases of elevated serum homocysteine level.
When the mutation is present in a heterozygous form, the concentration of homocysteine is not or is only slightly elevated in the serum, while this increase is much more explicit in the homozygous form of the mutation. The prevalence of the homozygous mutation in the population is relatively high; it is present in 5-15% of the average population.
FREQUENTLY ASKED QUESTIONS
- reducing or stopping smoking
- including more physical exercise in your daily routine
- having regular medical check-ups
- reducing the consumption of food rich in cholesterol

Contraceptives and the Leiden mutation
If you are carrying a Leiden mutation and you are taking contraceptive pills, then the 8-fold risk may rise to even 30-40-fold. If both of your alleles are mutant, taking contraceptives increases this risk from 80-fold to more than 100-fold Thus, it is recommended to have the test done before starting to take contraceptive pills.
Lactose intolerance
Lactose intoleranceLactose, present in milk, is degraded by the lactase enzyme in the small intestine. The amount of this enzyme is at its peak at birth and gradually decreases with age; thus, the ability to degrade lactose also decreases. Undigested lactose is responsible for a wide range of digestive problems such as increase of free water content, rapid intestinal transit, and development of hydrogen. Lactose intolerance is in fact an evolutionarily archaic form because adult mammals do not have to digest lactose. Based on national data, approximately 35-40% of the Hungarian adult population is sensitive to lactose to some extent. However, a significant portion of the people can consume milk products throughout their life; members of northern European populations or of northwest areas of India still have a sufficient amount of lactase activity as adults. The reason of this is the presence of variants in two locations of the lactase gene, which influences the elimination of enzyme production. The significant variability between different populations can be accounted for by the different milk consumption habits. In people with lactose intolerance, typical symptoms develop following milk consumption (diarrhoea, stomach cramps and pains, flatulence, feeling sick), which cease or can be relieved by introducing a lactose-free diet.
We recommend testing lactose intolerance:
- in babies; they may have congenital lactose intolerance, which inhibits the degradation of even breast milk. It is essential to distinguish lactose intolerance from milk protein sensitivity in order to select appropriate nutrition.
- in those individuals who have digestive problems following milk or dairy product consumption.
- in those individuals who have a lactose-intolerant family member (since lactose intolerance is genetically determined).
- in those individuals who have digestive problems characteristic of lactose intolerance even without dairy product consumption. Hidden lactose present in processed food can cause problems in sensitive individuals.
Early diagnosis is important because:
- besides causing unpleasant symptoms, lactose consumption continuously damages the absorptive surface of the small intestine
- other nutrients cannot be absorbed either, leading to their deficiency.
- in the long run, severe, irreversible diseases may develop (e.g. osteoporosis) due to the extensive damage in the absorptive surface of the small intestine.
- lactose-degrading bacteria create an acidic environment, which is associated with malignant tumours of the large intestine.

